Bonding Pairs And Lone Pairs Of Electrons In Carbon Dioxide
The option in co2 is to make two double bonds. Identify the compound with the highest percent ionic character.
8 answer the following questions based on the lewis dot structure for carbon dioxide on the right.
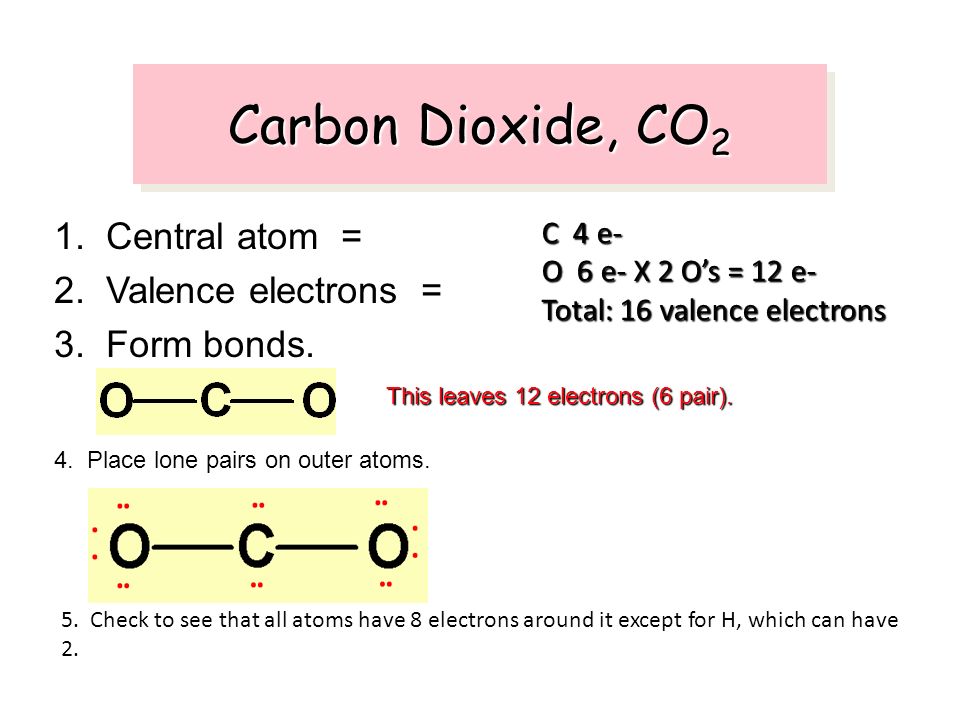
Bonding pairs and lone pairs of electrons in carbon dioxide. Yes because it is a tetrahedron. The electrons of the lone pair belong to the same atom. Carbon dioxide is a polar molecule whose positive center is on the carbon atom.
Determine the molecular formula for a compound that is 70 79 carbon 8 91 hydrogen 4 59 nitrogen and 15 72 oxygen. This atom will be 2sp hybridized with remaining 2p x and 2p y atomic orbitals. C is the water molecule polar.
Two on each oxygen. Why or why not. Therefore a lone pair is also called a non bonding electron pair although electrons in the innermost shells are also coupled and do not participate in the bonding they are not considered as lone pairs.
There are however two ways to do this. In order to find out how many lone pairs are in the. The carbon is happy making up to 4 bonds.
Lone pair is a pair of electrons that are not in a bond. It needs to gain 4 electrons and it has 4 electrons to contribute covalently. This positive center is able to attract and accept the lone electron pairs present on the oxide ion o 2.
Identify the number of bonding pairs and lone pairs of electrons in water. Bonding in carbon dioxide from the lewis structure we can see that the carbon in co 2 must make 2 sigma bonds and it has no lone pairs. Thus carbon dioxide is acting as a lewis acid and the oxide ion is acting as a lewis base.
A ibr b hcl c hf. A molecule of co2 contains one atom of carbon and two atoms of oxygen forming a compound or molecule pure substance of carbon dioxide. 2 bonding pairs and 2 lone pairs.
There are four lone pairs on carbon dioxide. To give the carbon atom an octet of electrons we can convert two of the lone pairs on the oxygen atoms to bonding electron pairs. What is a lone pair.
We can either take one electron pair from each oxygen to form a symmetrical structure or take both electron pairs from a single oxygen atom to give an asymmetrical structure. Lone pairs need to be part of the initial count of electrons prior to bonding. Atoms do not just gain lone pairs of electrons for no reason.
Each oxygen makes 1 sigma bond and also needs 2 orbitals for lone pairs of electrons. Each oxygen is bonded to the central carbon stom with a double bond so each oxygen needs two lone pairs to complete it s octet. It can be explained because of the tetrahedral arrangement around the oxygen and the presence of lone pairs electrons.
4 points a why does carbon dioxide have a.
Co2 Molecular Geometry And Bond Angles Carbon Dioxide Youtube
Interactions Between Carbon Monoxide Molecular Orbitals And Metal
Why So Math 2 Math Has Bent Structure While Co Math 2
Covalent Bonding Chapter Ppt Video Online Download
Chapter 8 Basic Concepts Of Chemical Bonding 8bonding Theories
Co2 Molecular Geometry And Lewis Structure
Chemical Bonding Water Cocaine Chapter Ppt Video Online Download
Ch4 Molecular Geometry W Free Video Guide
Title Lesson 4 Molecular Shapes Ppt Video Online Download
Co2 Molecular Geometry And Bond Angles Carbon Dioxide Youtube
Geometry Of Molecules Chemistry Libretexts
2 5 1 Electron Pair Repulsion Theory
Bond And Lone Pairs Valence Electrons Are Distributed As Shared Or
Chemical Bonding And Molecular Orbitals
Solved Below Is The Lewis Structure Of The Acetylene C2h
Vsepr For 2 Electron Clouds Video Khan Academy
Solved How Many Lone Pairs Of Electrons Are Assigned To C
Solved How Many Lone Pairs Of Electrons Arc Assigned To T
Lone Pair On Carbon In Co2 Chemistry Stack Exchange
Shapes Of Molecules Online Presentation
How Many Pairs Of Electrons Should Be Drawn In The Lewis Structure
Covalent Bonding Chapter Ppt Video Online Download
Carbon Dioxide Has A Linear Molecular Geometry Carbon At The
5 2 Molecular Shape Chemistry Libretexts
Video Drawing A Lewis Structure For Carbon Dioxide Co Nagwa
Chapter 10 Properties Of Solids And Liquids Molecular Structures
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcqordlvxkztarqhfqaimg5pkpjtotnukvqppzwoyl1tkbomoew3 Usqp Cau
Solved Chemical Bonding Counting Electron Pairs In A Lewi
Solved 1 Identify The Number Of Bonding Pairs And Lone P
Is Co2 Linear While So2 Is Bent Quora
Posting Komentar
Posting Komentar