Purpose Of Salt Bridge In Galvanic Cell
A salt bridge in electrochemistry is a laboratory device used to connect the oxidation and reduction half cells of a galvanic cell voltaic cell a type of electrochemical cell. Before the wires are connected the solutions in each beaker are.
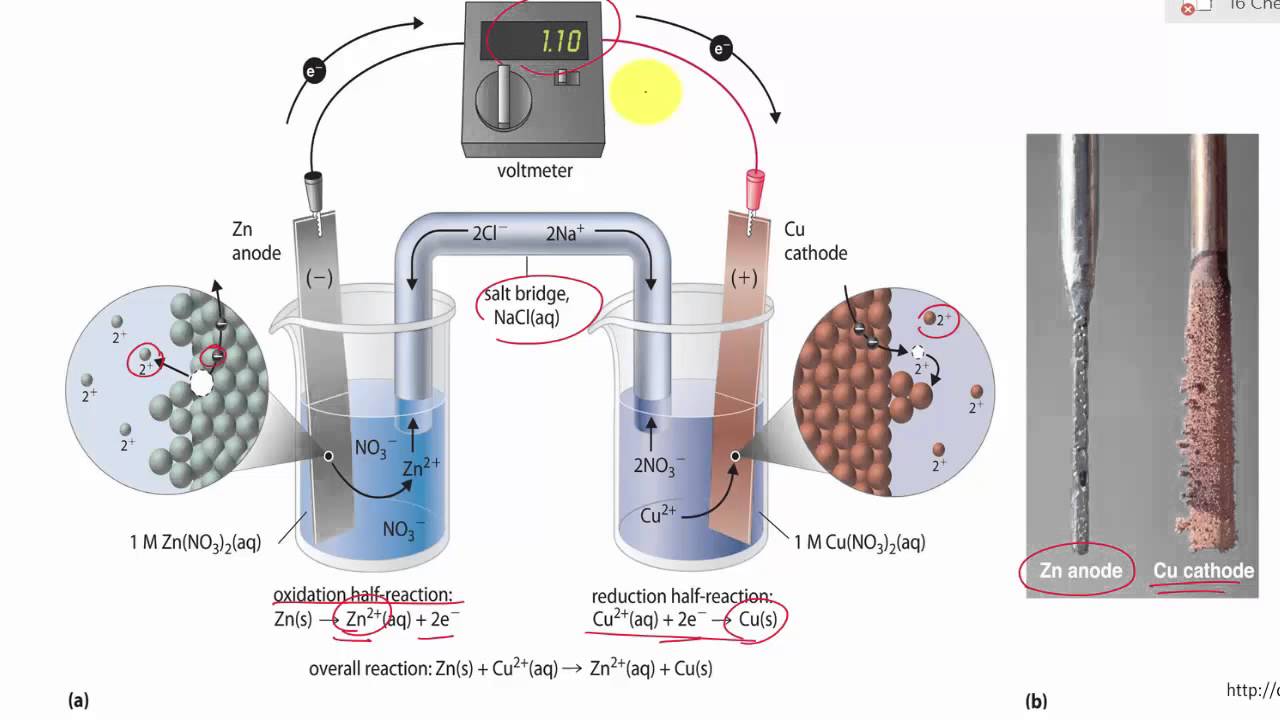 Describe The Function Of A Salt Bridge Youtube
Describe The Function Of A Salt Bridge Youtube
It maintains electrical neutrality within the internal circuit preventing the cell from rapidly running its reaction to equilibrium.
Purpose of salt bridge in galvanic cell. Spontaneous what is the purpose of a salt bridge in a galvanic voltaic cell. A salt bridge in electrochemistry is a laboratory device used to connect the oxidation and reduction half cells of a galvanic cell voltaic cell a type of electrochemical cell. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly.
For example agno 3 kcl etc. The buildup of charges within the cells will result in negative feedback slowing down the reaction if not for the salt bridge. Salt bridges are generally used in a galvanic cell such as a voltaic cell or daniel cell.
A salt bridge is a u shaped tube containing concentrated solution of an inert electrolyte like kcl kno3 k2so4 etc. The oxidation reaction that occurs at the anode generates electrons and positively charged ions. It maintains electrical neutrality within the internal circuit preventing the cell from rapidly running its reaction to equilibrium.
Let s see what happens without a salt bridge in a galvanic cell. Or solidified solution of such an electrolyte in agar agar. A salt bridge is a connection containing a weak electrolyte between the oxidation and reduction half cells in a galvanic cell e g voltaic cell daniell cell.
If no salt bridge were present the solution in one half cell would accumulate negative charge and the solution in the other half cell would accumulate positive charge as the reaction proceeded quick. The electrons flow from the anode to the cathode. An inert electrolyte is one whose ions do not take part in the redo.
A salt bridge is a pathway for ions to flow between the beakers. The salt bridge permits negative charged ions in the cathode cells to flow to the anode cell and vice versa. The salt bridge usually consists of a strong electrolyte which is further made up of ions.
The purpose of a salt bridge is not to move electrons from the electrolyte rather it s to maintain charge balance because the electrons are moving from one half cell to the other. A salt bridge in electrochemistry is a laboratory device used to connect the oxidation and reduction half cells of a galvanic cell a type of electrochemical cell. The loss of positive ions in the cell results in a net negative charge.
Exchange anions and cations to balance dissipate newly generated charges and preventing them from building up and halting the reaction what is the order of the cell diagram for a galvanic voltaic cell. The main function of a salt bridge is to help maintain the electrical neutrality within the internal circuit. It maintains electrical neutrality within the internal circuit preventing the cell from rapidly running its reaction to equilibrium.
Does The Absence Of A Salt Bridge In A Galvanic Cell Stop The
 Electrochemical Cells Batteries Ppt Video Online Download
Electrochemical Cells Batteries Ppt Video Online Download
 Kac32 17 Electrochemistry The Role Of The Salt Bridge Youtube
Kac32 17 Electrochemistry The Role Of The Salt Bridge Youtube
Schematic Diagram Of A Potentiometric Electrochemical Cell Image
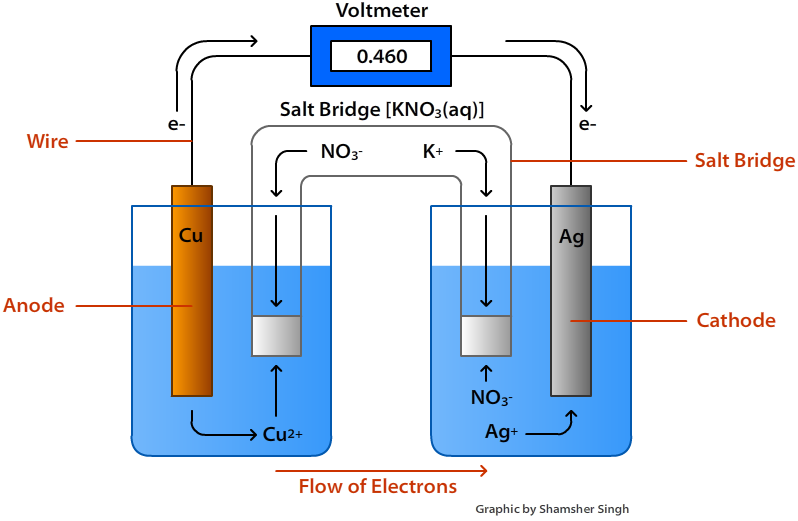 Voltaic Cells Chemistry Libretexts
Voltaic Cells Chemistry Libretexts
 What Is The Purpose Of Salt Bridge Quora
What Is The Purpose Of Salt Bridge Quora
 Do Ions In A Salt Bridge Come From The Half Cells In
Do Ions In A Salt Bridge Come From The Half Cells In
 Why Is It Important To Use A Salt Bridge In A Voltaic Cell Can A
Why Is It Important To Use A Salt Bridge In A Voltaic Cell Can A
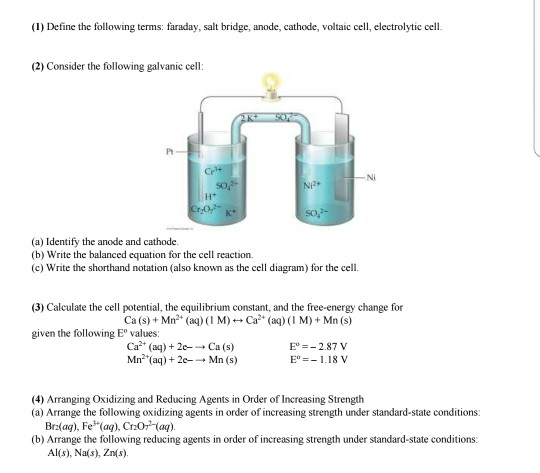
 Solved 15 The Purpose Of The Salt Bridge In An Electroch
Solved 15 The Purpose Of The Salt Bridge In An Electroch
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcqgbtopxb9gmn99axdk99qqow2qm6pvajavlz3rgkbpxvhcccce Usqp Cau
 Understanding Purpose Of Salt Bridge Galvanic Cell Chemistry
Understanding Purpose Of Salt Bridge Galvanic Cell Chemistry
 Electrochemical Cell Definition Description Types Applications
Electrochemical Cell Definition Description Types Applications
 Solved What Is The Purpose Of A Salt Bridge In An Electro
Solved What Is The Purpose Of A Salt Bridge In An Electro
 Electrolytic Cell An Overview Sciencedirect Topics
Electrolytic Cell An Overview Sciencedirect Topics
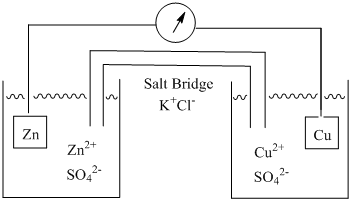 5 Electrochemical Cells Chemistry Libretexts
5 Electrochemical Cells Chemistry Libretexts
 Galvanic Cells Chemistry For Majors
Galvanic Cells Chemistry For Majors
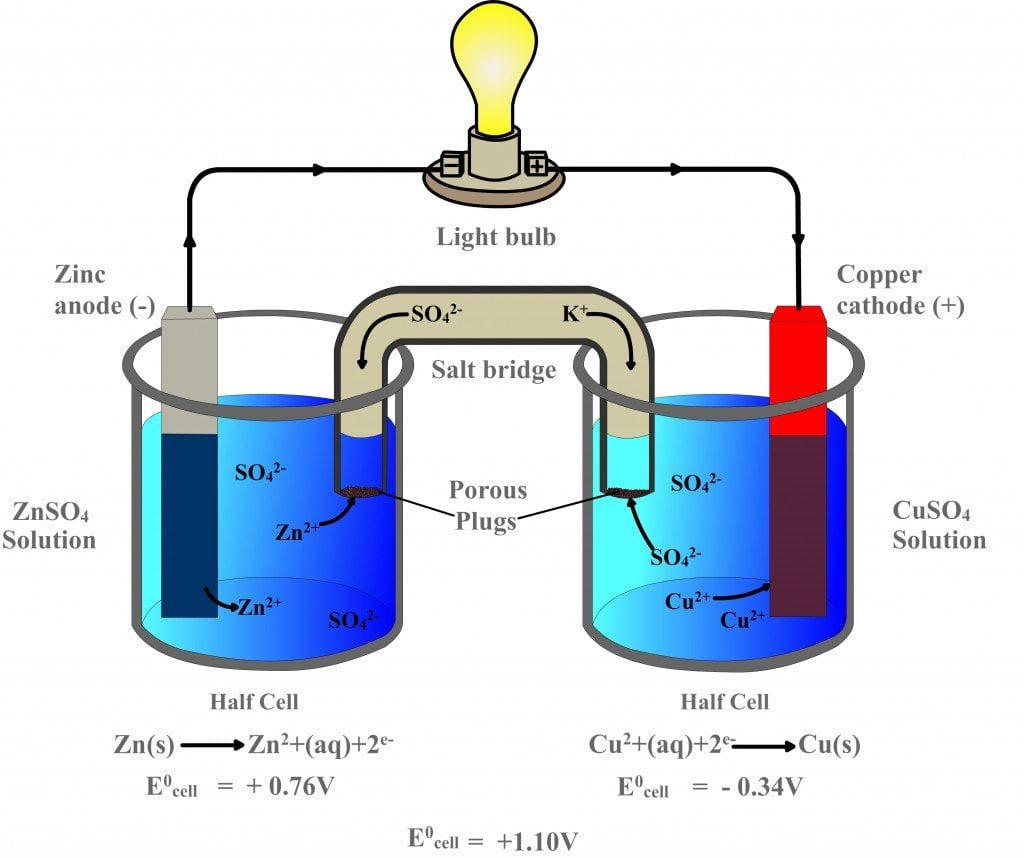 Galvanic Cell Definition Diagram And Working
Galvanic Cell Definition Diagram And Working
How Does A Salt Bridge Work In Electrolysis Quora
 Galvanic Cell Blue Png Download 831 666 Free Transparent
Galvanic Cell Blue Png Download 831 666 Free Transparent
Galvanic Cells Galvanic Cells Sparknotes
 Introduction To Electrochemistry By T Hara
Introduction To Electrochemistry By T Hara
 What Is The Purpose Of Salt Bridge Quora
What Is The Purpose Of Salt Bridge Quora
 Ch 20 Electrochemistry Lecture 2 Galvanic Cells Ppt Download
Ch 20 Electrochemistry Lecture 2 Galvanic Cells Ppt Download
 Electrochemical Salt Bridge Function Preparation Study Com
Electrochemical Salt Bridge Function Preparation Study Com
 Difference Between Galvanic Cell And Concentration Cell Compare
Difference Between Galvanic Cell And Concentration Cell Compare
 Electrochemical Salt Bridge Definition Purpose Video Lesson
Electrochemical Salt Bridge Definition Purpose Video Lesson
 How Does A Salt Bridge In A Galvanic Cell Neutralize Each Half
How Does A Salt Bridge In A Galvanic Cell Neutralize Each Half
 Why Salt Bridge Are Used In Galvanic Cell Quora
Why Salt Bridge Are Used In Galvanic Cell Quora
Http Chemistrybook2011 Blogspot Com 2011 05 Function Of Salt Bridge Html
/saltbridge-5af43fcf875db900368d1853.jpg)
/saltbridge-5af43fcf875db900368d1853.jpg)
/saltbridge-5af43fcf875db900368d1853.jpg)

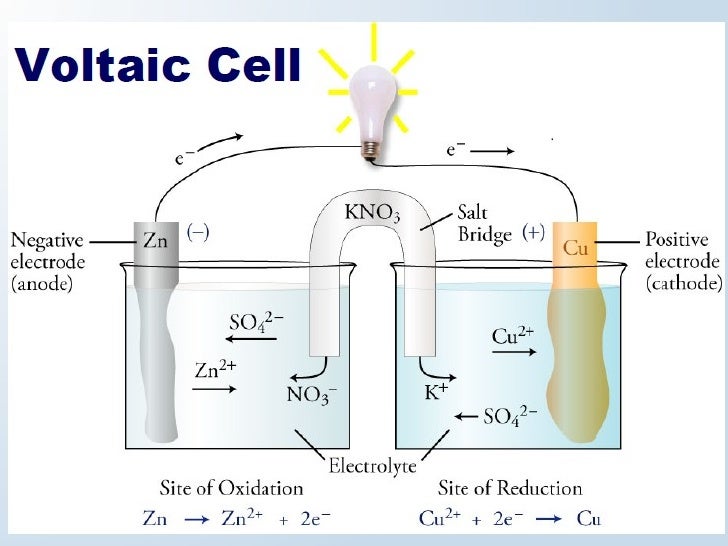
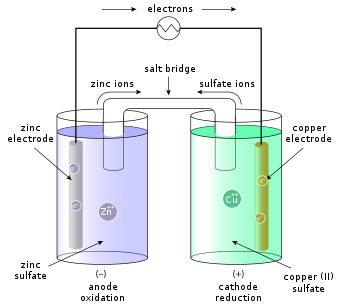
Posting Komentar
Posting Komentar