Ph Of Weak Acid And Strong Base Formula
Instead their ionization is a two way reaction with a definite equilibrium point. The ph equation is still the same ph log h but you need to use the acid dissociation constant ka to find h.
Lab 6 Mixtures Of Acids And Bases
So a base based on some other mechanism such as nh 3 which does not contain oh ions as part of its formula will be a weak base.
Ph of weak acid and strong base formula. Mixture of a strong acid and a strong base. C 5 h 5 n. These include the initial ph the ph after adding a small amount of base the ph at the half neutralization the ph at the equivalence point and finally the ph after adding excess base.
Identifying strong and weak acids and bases. There are two main methods of solving for hydrogen ion concentration. At 25 c solutions with a ph less than 7 are acidic and solutions with a ph greater than 7 are basic.
When solving a titration problem with a weak acid and a strong base there are certain values that you want to attain. Finding the ph of a weak acid is a bit more complicated than finding ph of a strong acid because the acid does not fully dissociate into its ions. The ph of a weak base falls somewhere between 7 and 10.
This page consist of method formula and solved examples to calculate ph of salts of weak acids and strong bases. Identify each acid or base as strong or weak. On mixing a strong acid and strong base neutralization ph 7 takes place.
The ph value can be less than 0 for very strong acids or greater than 14 for very strong bases. The neutral value of the ph depends on the temperature being lower than 7 if the temperature increases. The ph of a 2 00 m solution of a strong acid would be equal to log 2 00 0 30.
The procedure for calculating the ph of a solution of a weak base is similar to that of the weak acid in the sample problem. Like weak acids weak bases do not undergo complete dissociation. The higher ph of the 2 00 m nitrous acid is consistent with it being a weak acid and therefore not as acidic as a strong acid would be.
Say n1 v1 is the strength and volume of the strong acid and n2 v2 is the strength and volume of the strong base. The resulting solution may be an acid or base depending on the concentration. Weak acid and strong base titration problems.
While strong bases release hydroxide ions via dissociation weak bases generate hydroxide ions by reacting with water.
Weak Acid Strong Base Titration Problems Ph Calculations
Monoprotic Acid Base Equilibria Ppt Download
Salt Hydrolysis Study Material For Iit Jee Askiitians
Salt Hydrolysis Of Weak Acid And Weak Base And Ph Formula Hindi
Weak Acid Strong Base Titration Ph At Equivalence Point Youtube
Titration Of A Weak Acid With A Strong Base Chemistry Libretexts
How To Calculate The Dissociation Constant Of A Weak Acid From The
Pharmaceutical Analytical Chemistry Ppt Video Online Download
Titration Of A Weak Acid With A Strong Base Video Khan Academy
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcscdaiz9ewmqfnz1aft3d6bkbvw4oc 1sfbg1p7mb9 Mn4z8uyc Usqp Cau
Acid Base Titrations And Buffered Solutions
Weak Acid Strong Base Calculations Ppt Video Online Download
Weak Acid Strong Base Titrations
Solved 3 Weak Base Strong Acid Titration Use Approximat
When Adding Base To A Weak Acid Does The Concentration Of Ha
Strong Acid Strong Base Titration Problems With Ph Calculations
Acid Base Titrations Boundless Chemistry
Acid Base Titration Curves Ph Calculations Weak Strong
Titration Curve For Weak Acid Strong Base
Titration Curves Equivalence Point Article Khan Academy
Solved In The Titration Of A Strong Acid With A Strong Ba
Calculation Of The Ph Of A Mixture Of A Strong Acid And Weak Acid
13 3 Finding The Ph Of Weak Acids Bases And Salts Chemistry
Calculation Of Hydrolysis Constant Degree Of Hydrolysis And Ph Of
Ph Curves Titrations And Indicators Secondary Science 4 All
Weak Acid Strong Base Titrations
Titration Of A Weak Base With A Strong Acid Chemistry Libretexts
Titration Of A Weak Base With A Strong Acid Video Khan Academy
Titration Chemistry For Non Majors
Strength Of Acids Boundless Chemistry
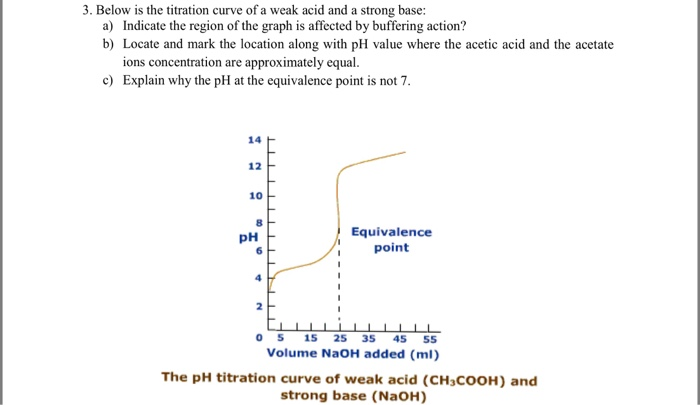
Solved Below Is The Titration Curve Of A Weak Acid And A
Weak Acid Strong Base Titration Ph After Equivalence Point
Ph Calculation For Weak Acids And Bases Youtube
Posting Komentar
Posting Komentar