Coordinate Covalent Bond Examples Lewis Structure
Langmuir in 1919 improved the lewis concept by suggesting that when both the atoms taking part in a chemical combination are short of electrons than the nearest noble gas configuration they can share their electrons in order to complete their octet. Using lewis dot symbols to describe covalent bonding the valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure stoichiometry and properties.
Corechem An Excess Of Bonds Chemprime
For example chlorine with seven valence electrons is one electron short of an octet.
Coordinate covalent bond examples lewis structure. For example below two hydrogen atoms each with a single electron can share their electrons to form a covalent bond and create the diatomic hydrogen molecule. Metal ligand interactions in most organometallic compounds and most coordination compounds are described similarly. Writing lewis structures for polyatomic ions recall that a polyatomic ion is a group of atoms that are covalently bonded together and which carry an overall electrical charge.
Coordinate covalent bonding is pervasive. In this molecular state both individual hydrogen atoms attain the noble gas configuration of helium. In all metal aquo complexes m h 2 o n m the bonding between water and the metal cation is described as a coordinate covalent bond.
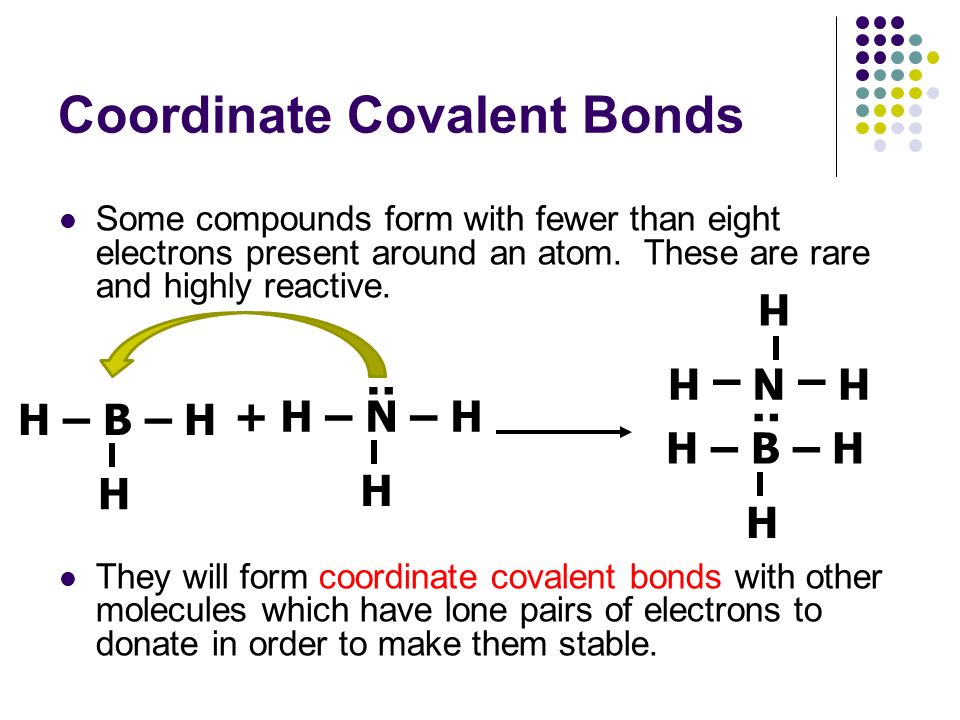
Covalent bond lewis langmuir concept. Formation of an adduct of ammonia and boron trifluoride involving formation of a coordinate covalent bond. The ammonium ion nh 4 is formed when a hydrogen ion h attaches to the lone pair of an ammonia nh 3 molecule in a coordinate covalent bond.
If a species atom or ion could react with a water molecule in such a way that the oxygen atom shared both the electrons in one of its lone pairs with the other species without this other species sharing any electrons with the oxygen atom then a covalent bond would form and this covalent bond would be called a coordinate covalent bond or a dative bond.
Dative Bond Definition Examples How To Identify
What Is A Coordinate Covalent Bond Youtube
Lewis Dot Symbols And Lewis Structures Boundless Chemistry
Lewis Dot Structure Flow Chart Wikispaces
Coordinate Bonds Brilliant Math Science Wiki
Video Identifying Dative Covalent Bonds Nagwa
Lewis Structures Of So2 Co3 No2 O3 And More Dative Bonding
Co Ordinate Bond Definition Examples Formation
Co Ordinate Bond Definition Examples Formation
Coordinate Covalent Bonds And Resonance Structures Flashcards
Covalent Bonds Bonding And Chemical Interactions Training Mcat
Covalent Bonding Lewis Structures Ppt Video Online Download
9 5 Covalent Bonding Lewis Structure Chemistry Libretexts
Chemistry Chemical Bonding 20 Of 35 Lewis Structures For Ions
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctma4ii0rfwqclqy2dd7yue6 Xkm1sdrxnri88utnchy8bm Fwo Usqp Cau
Coordinate Covalent Bond Wikiwand
Coordinate Bond Chemistry Britannica
Covalent Bond An Overview Sciencedirect Topics
Co Ordinate Dative Covalent Bonding
Lewis Structures And Covalent Bonding
Dative Covalent Bonds Year 12 Chemistry
Identify The Coordinate Covalent Bond S Present If Any In Each
Coordinate Dative Covalent Bonding Chemistry Libretexts
Mcat Ordinary Covalent Bond Vs Coordinate Covalent Bond Youtube
Aim What Are Coordinate Covalent Bonds Do Now Finish The
What Is A Coordinate Covalent Bond Youtube
Coordinate Covalent Bond Wikipedia
Coordinate Bonds Brilliant Math Science Wiki
With The Assumption That The Ammonium Ion Is Formed From The
Ib Chemistry Year 1 Hl The Brooklyn Latin School Ppt Download
4 3 Coordinate Covalent Bonds Sl Youtube
What Is The Difference Between Covalent Bond And Coordinate
Co Ordinate Dative Covalent Bonding
Posting Komentar
Posting Komentar