Oxidation States Of Carbon Atoms
Assigning oxidation numbers to organic compounds the oxidation state of any chemically bonded carbon may be assigned by adding 1 for each more electropositive atom h na ca b and 1 for each more electronegative atom o cl n p and 0 for each carbon. This means that every c h bond will decrease the oxidation state of carbon by 1.
A Level Redox 3 Oxidation Reduction Organic Chemistry
This means that the carbon atoms in fatty acids have more electrons around them.
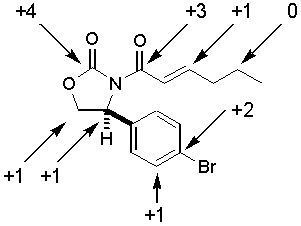
Oxidation states of carbon atoms. Predict the product for the following reaction sequence. Any two bonds between the same atom do not affect the oxidation state recall that the oxidation state of cl in cl cl and that of h in h h is zero. Such as the golden tio blue black ruo 2 or coppery reo 3 all of obvious oxidation state ultimately however the assignment of the free metallic electrons to one of the bonded atoms has its limits and leads to unusual oxidation states.
How to assign oxidation states to carbon in different compounds. Predict the product for the following reaction. Notice how all the carbon atoms in glucose a sugar have oxidation states of 1 0 or 1 while all but one carbon atom in palmitic acid a fatty acid have oxidation states of 2 or 3.
For carbon bonded to a more electronegative non metal x such as nitrogen oxygen sulfur or the halogens each c x bond will increase the oxidation state of the carbon by 1. These examples will use the rules outlined in. Atoms within a molecule are held together by the force of attraction that the nuclei of two or more of them exert on electrons in the space between them.
Oxidation states are assigned to atoms by a set of rules based on the arrangement of electrons and bonds around that atom. So a carbon attached to 4 carbons has an oxidation state of zero. In a c h bond the h is treated as if it has an oxidation state of 1.
Many compounds with luster and electrical conductivity maintain a simple stoichiometric formula. Now here s a fun exercise. Provide the reagents necessary to carry out the following conversion.
Try applying the same rules to carbon. So compounds like feo and fe o were called ferrous and ferric oxides while sno and sno were called stannous and stannic oxides. The idea of assigning an oxidation state to each of the atoms in a molecule evolved from the electron pair concept of the chemical bond.
Oxidation originally referred to reactions with oxygen. Predict the product for the following reaction. This means each atom in the molecule has its own oxidation state which could be different from similar atoms in the same molecule.
What is the oxidation state of the carbon atoms i and ii in the following reaction. If the compound has a charge you adjust the oxidation states accordingly so that their sum equals the charge. Oxidation state in metals.
The algebraic sum of the oxidation states in an ion is equal to the charge on the ion. If the compound is neutral the sum of the oxidation states also has to be neutral. This means that every c h bond will decrease the oxidation state of carbon by 1.
Certain non metals are less electronegative than carbon such as. Calculating oxidation states in inorganic compounds. The oxidation state of an atom in a molecule refers to the degree of oxidation of that atom.
Oxidation reduction reaction oxidation reduction reaction oxidation states.
Common Classes Of Organic Reactions
Solution What Is The Oxidation State Of Chemistry
What Is The Oxidation Number Of Carbon In Ch3cooh Quora
Oxidation States Of Carbon Atoms In Diamond And Graphite Are
19 1 Definition Of Oxidation State For Carbon Organic Chemistry Ii
Calculating The Oxidation State Of A Carbon Master Organic Chemistry
Solved 1 5 Circle The Oxidation State Of The Carbon Atom
Solved What Is The Oxidation State Of The Carbon Atoms I
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrhwbm6t0xbhwykrr1atetq Lw2e1counkwywoqgadbvfr Cqva Usqp Cau
Oxidation Numbers Of Carbon Compounds With One Carbon Atom Youtube
Oxidation And Reduction In Organic Chemistry Master Organic
Oxidation States Of Organic Molecules Chemistry Libretexts
Solved Testbank Question 056 What Are The Oxidation Stat
Ppt Oxidation Of Monosaccharides Powerpoint Presentation Free
Oxidation States In Sugar Reactions
Carbon Oxidation State Osc Vs Number Of Carbon Atoms For
Solved What Is The Oxidation State Of The Carbon Atoms I
I Thought Metals Only Had Oxidation States
What Is The Oxidation Number Of Each Carbon In Glucose Quora
19 1 Definition Of Oxidation State For Carbon Organic Chemistry Ii
Average Oxidation States Of Carbon And Number Of Carbon Atoms Of
Is Esterification A Redox Reaction Anhourofchemaday
Counting Oxidation States Amphoteros
Calculating The Oxidation State Of A Carbon Master Organic Chemistry
19 1 Definition Of Oxidation State For Carbon Organic Chemistry Ii
Ib Chemistry On Redox Oxidation States And Oxidation Number
Organic Oxidation Reduction Reactions Video Khan Academy
Solved What Is The Oxidation State Of The Carbon Atoms I
Oxidation States In Sugar Reactions
Posting Komentar
Posting Komentar