Example Of London Dispersion Forces
London dispersion forces example the unequal distribution of electrons about the nucleus in an atom can induce some dipole in the atom. The forces are used to explain the universal attraction between bodies the physical adsorption of gases and the cohesion of condensed phases.
London Dispersion Forces Youtube
London forces occur in both nonpolar and polar molecules and can affect a chemical compound s physical state.
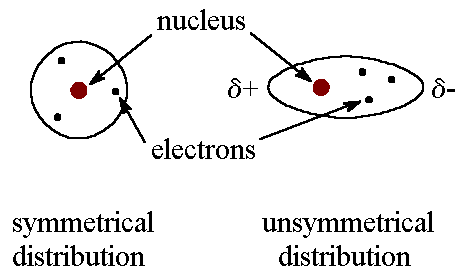
Example of london dispersion forces. Dispersion forces are long range and can be effective from large distance 10nm down to interatomic distances. They are the weakest of the intermolecular forces but strengthen as the atoms at the source of the forces increase in size. At room temperature neopentane c 5 h 12 is a gas whereas n pentane c 5 h 12 is a liquid.
Chlorine is an example of an atom whose interactions are shaped by these london forces. Stronger between molecules that are easily polarized. The van der waals forces encompass intermolecular forces as well as some intramolecular forces including keesom interaction the debye force and the london dispersion force.
Even though it is weak of the three van der waals forces orientation induction and dispersion the dispersion forces are usually dominant. Dispersion forces may be repulsive or attractive. A dipole exists when part of the molecule is has a net positive charge and another part has a net negative charge.
These london dispersion forces are often found in the halogens e g f 2 and i 2 the noble gases e g ne and ar and in other non polar molecules such as carbon dioxide and methane. Weaker between molecules that are not easily polarized. These london dispersion forces are often found in the halogens e g f 2 and i 2 the noble gases e g ne and ar and in other non polar molecules such as carbon dioxide and methane.
London dispersion forces are part of the van der waals forces or weak intermolecular attractions. Methane is a non polar molecule meaning there is no buildup of negative or positive charge anywhere on the molecule. The london dispersion force is the weakest of the van der waals forces and is the force that causes nonpolar atoms or molecules to condense into liquids or solids as the temperature is lowered.
The principle aspect of dispersion force is the determination of the order of magnitude of the attractive force. Molecular shape the shapes of molecules also affect the magnitudes of dispersion forces between them. Liquid methane gas ch4 would be an example of london dispersion forces.
London dispersion forces tend to be. London dispersion forces are part of the van der waals forces or weak intermolecular attractions. London dispersion forces named after german american physicist fritz london are one of the three van der waals intermolecular forces holding molecules together.
The main features of dispersion force london dispersion force is. When another atom or molecule comes in contact with this induced dipole it can be distorted that leads to an electrostatic attraction between either atoms or molecules.
London Dispersion Forces Tutorial Sophia Learning
A Simple Explanation Of Intermolecular Forces With Examples
Types Of Intermolecular Forces
Intermolecular Forces Hydrogen Bonding Dipole Dipole Ion
Intramolecular And Intermolecular Forces Article Khan Academy
Identifying London Dispersion Forces Youtube
London Dispersion Forces In Sterically Crowded Inorganic And
12 6 Intermolecular Forces Dispersion Dipole Dipole Hydrogen
London Dispersion Forces Temporary Dipole Induced Dipole
10 1 Intermolecular Forces General College Chemistry I
Intramolecular And Intermolecular Forces Article Khan Academy
London Dispersion Force Is A Temporary Attractive Force That
Ppt What Are Dispersion Forces Powerpoint Presentation Free
Ppt Chapter 11 Liquids And Intermolecular Forces Powerpoint
London Dispersion Forces Youtube
Intermolecular Forces Boundless Chemistry
London Dispersion Forces Van Der Waals Forces Weak
Are Dipole Dipole Forces Or London Dispersion Forces Stronger
In What Case Are London Forces Stronger Than Dipole Forces Quora
Dublin Schools Lesson London Dispersion Forces
Intermolecular Forces Notes Intermolecular Forces O
Imf Part 1 London Dispersion Forces Youtube
Intermolecular Forces Notes Ppt Download
London Dispersion Forces Definition Examples Formula Van Der
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsfndjwrf5szdkf0usqec6vxmu6z3x9n4ixppby5rz9c1dihopl Usqp Cau
Intermolecular Forces London Dispersion Forces And Dipole Dipole Att
Van Der Waals Force Facts Definition Dispersion
Intermolecular Forces Boundless Chemistry
London Dispersion Force Wikipedia
Intermolecular Bonds Chemistry Socratic
Dispersion Forces Intermolecular Forces
Intramolecular And Intermolecular Forces Article Khan Academy
Intermolecular Forces London Dispersion Forces And Dipole Dipole Att
London Dispersion Forces Van Der Waals Forces Weak
Van Der Waals London Dispersion Forces The Credible Hulk
The Four Intermolecular Forces And How They Affect Boiling Points
Posting Komentar
Posting Komentar