Why Does Electronegativity Increase Across A Period
Think of sodium chloride as if it were covalently bonded. So as you move down a group on the periodic table the electronegativity of an element decreases because the increased number of energy levels puts the outer electrons very far away from the pull of the nucleus.
Periodic Behavior Presentation Chemistry
Measure the bond strength increase get a chi difference.
Why does electronegativity increase across a period. Fluorine is the most electronegative element. Both sodium and chlorine have their bonding electrons in the 3 level. Think of sodium chloride as if it were covalently bonded.
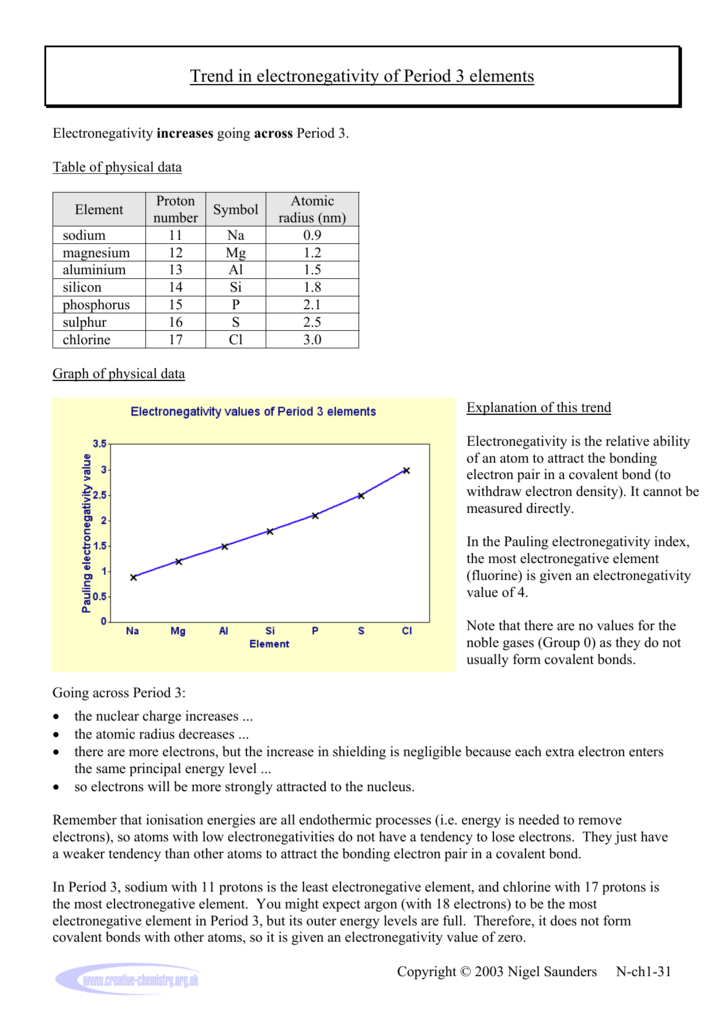
Across a period from left to right the electronegativity of atoms increases. Electronegativity increases as you move across the periodic table from left to right. Consider sodium at the beginning of period 3 and chlorine at the end ignoring the noble gas argon.
Why does electronegativity increase across a period. Well the electronegativity increases across the period because the electrons are being added onto the same energy level this increases the number of electrons of an atom the increase of electrons of an atom also leads to increase in clear charge. There are a few reasons why electronegativity values increase while going across periods.
Why does electronegativity increase across a period. Electronegativity is a measure of an atom s attraction for the electrons in a bond. However it does not form covalent bonds so it does not have an electronegativity value.
Why does electronegativity increase across a period. In period 3 sodium with 11 protons is the least electronegative element and chlorine with 17 protons is the most electronegative element. Both sodium and chlorine have their bonding electrons in the 3 level.
Why does electronegativity increase as you move across a period. Consider sodium at the beginning of period 3 and chlorine at the end ignoring the noble gas argon. Electronegativity is the ability of an atom in a molecule to attract shared electrons to itself.
So for the pauling scale your question becomes why does moving across a period result in greater delta minus character in a covalent bond. This occurs due to a greater charge on the nucleus causing the electron bonding pairs to be very attracted to atoms placed further right on the periodic table. Ask question asked 6 years 7 months ago.
You might expect argon with 18 electrons to be the most electronegative element in period 3. Turns out the answer is the same increased zeff and lower e orbitals.
Ionic And Covalent Bonding Electronegativity General Chemistry
Definition Of Electronegativity Chegg Com
Physical Properties Of Period 3 Elements Secondary Science 4 All
What Is Electronegativity Trend Example Education Career
Chemistry Coach John Periodic Law Flashcards Quizlet
Periodic Trends Chemistry Libretexts
Trends Of Periodic Atomic Properties
B Periodicity After Completing This Topic You Should Be Able To
Trend In Electronegativity Of Period 3 Elements
Electronegativity Difference Broadneck High School
Why Is Si Silicon More Electronegative Than P Phosphorus Quora
Why Does Electronegativity Increase From Left To Right Across The
Why Does Electronegativity Increase Across A Period And Decrease
Electronegativity Trends Among Groups And Periods Of The Periodic
How Does Electronegativity Change Across A Period Socratic
What How Why Radius Electronegativity Ionization Energy Ppt
Physics Page Electronegativity Is A Measure Of The Facebook
Https Southhoustonhs Pasadenaisd Org Common Pages Displayfile Aspx Itemid 2286084
Electronegativity Chart List Of Electronegativity
Periodic Trends In Electronegativity Ck 12 Foundation
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcq12jbwdu66cjtcoixganw4qzmoqwoucv5l Yvdu1fzhnwkbsmk Usqp Cau
Topic 3 Periodicity Ppt Video Online Download
Solved What Is The General Trend In Electronegativity Acr
Ppt Catalyst 9 6 13 Powerpoint Presentation Free Download
Periodic Trends In Electronegativity Ck 12 Foundation
Change In Electronegativity Order Down A Group From Groups 13 16
Electronegativity Flashcards Quizlet
Trends In The Periodic Table Course Hero
Electronegativity Trends Among Groups And Periods Of The Periodic
3 2 Physical Properties Accelerated Study Notes
Why Does Electronegativity Decrease From Top To Bottom On Periodic
Physics Page Electronegativity Is A Measure Of The Facebook
Electronegativity Video Khan Academy
Posting Komentar
Posting Komentar