How To Make An Epoxide
In general low molecular weight epoxides are colourless and nonpolar and often volatile. An epoxide is a cyclic ether with a three atom ring.
Epoxide Chemical Compound Britannica
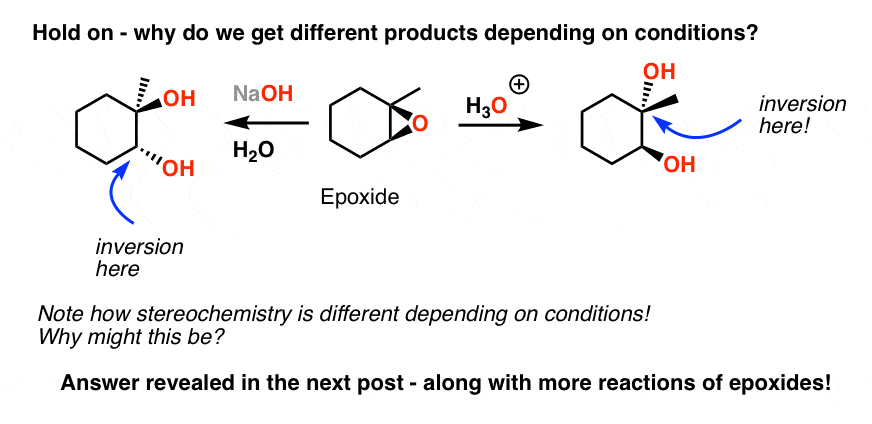
Write the mechanism for the opening of an epoxide ring by an aqueous acid paying particular attention to the stereochemistry of the product.
How to make an epoxide. Economically ethylene oxide is the most important epoxide and is created by oxidation of ethylene over a silver catalyst. Epoxide cyclic ether with a three membered ring. An epoxide is a 3 membered ring containing two carbon atoms and one oxygen atom.
Ethylene oxide is economically. C c to an epoxide. We ll explore this reaction of epoxides in more detail in subsequent posts.
Under the same conditions that open the epoxide diethyl ether is inert as are most ethers. The strain of the three membered ring makes an epoxide much more reactive than a typical acyclic ether. In the presence of a base ring closure occurs via an intramolecular s n 2 reaction.
Epoxide can be synthesized in many ways. Identify the product formed when an epoxide ring is opened by a hydrogen halide under anhydrous conditions. It is used as a fumigant and to make ethylene glycol antifreeze and other useful compounds.
This ring approximates an equilateral triangle which makes it strained and hence highly reactive more so than other ethers they are produced on a large scale for many applications. Suitable base could be hydroxide ho or maybe na metal. The basic structure of an epoxide contains an oxygen atom attached to two adjacent carbon atoms of a hydrocarbon.
2 2 2 trifluoroacetophenone is an efficient organocatalyst for a cheap mild fast and environmentally friendly epoxidation of alkenes. It is interesting because it is easily opened due to small ring strain and due to the electronegativity of the oxygen atom. Many students like to remember it as a cyclic ether.
The reagents and starting materials. Halohydrins can be formed by the addition reaction x 2 h 2 o or hox to alkenes. For example treating an epoxide with aqueous acid h 3 o will open an epoxide to provide a 1 2 diol often called a vicinal diol or a glycol.
Predict the major product from the acidic cleavage of a given unsymmetrical epoxide.
Epoxide Formation From Halohydrins Chemistryscore
Solved Grignard Reactions Are Often Limited By Steric Hin
Epoxide Synthesis By Epoxidation
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsszqqnzvleeos4or9ooqcbdqes9 O9oxl6cxvqbb Horjixq2s Usqp Cau
Organic Chemistry 17 Anchimeric Assistance Epoxide Transformations
Epoxides Formation And Utilization Organic Chemistry Help
Epoxides The Outlier Of The Ether Family Master Organic Chemistry
Epoxide Synthesis By Epoxidation
Epoxide Chemical Compound Britannica
Isomerization Of Epoxides Jat 2019 Advanced Synthesis Amp
Epoxides The Outlier Of The Ether Family Master Organic Chemistry
Epoxides The Outlier Of The Ether Family Master Organic Chemistry
Epoxides The Outlier Of The Ether Family Master Organic Chemistry
Epoxidation Of Alkenes With Free Study Guide
Synthesis Of An Epoxide From 1 2 Diol Chemistry Stack Exchange
The Mechanism Of How Halohydrins Make Epoxides Via Intramolecular
Solved 1 Another Mechanism For The Formation Of Epoxides
Epoxides The Outlier Of The Ether Family Master Organic Chemistry
Epoxides Formation And Utilization Organic Chemistry Help
Epoxides The Outlier Of The Ether Family Master Organic Chemistry
Epoxides The Outlier Of The Ether Family Master Organic Chemistry
Epoxide Ring Opening With Base Master Organic Chemistry
Epoxide Reactions Organic Chemistry Help
Epoxides The Outlier Of The Ether Family Master Organic Chemistry
Epoxides The Outlier Of The Ether Family Master Organic Chemistry
Epoxides Formation And Utilization Organic Chemistry Help
Ch 11 4 Epoxide Synthesis And Reactions
Ether And Epoxide Reactions Youtube
Posting Komentar
Posting Komentar